Thermal precision serves as a critical safeguard for preserving vaccine efficacy and protecting public health. Vaccines are among the most temperature-sensitive products in the global supply chain, and even minor deviations from specified storage conditions can result in irreversible degradation. As global immunization efforts intensify and the pharmaceutical landscape evolves to include more complex biologics, the margin for error continues to narrow. Ensuring thermal consistency across every stage of the supply chain is no longer a technical challenge; it is a matter of public health integrity.
Compounding this urgency is the scale and velocity of today’s vaccine distribution networks, which now span continents and operate under tight regulatory scrutiny. Cold chain failures not only risk product loss but also delay immunization campaigns, erode public trust, and expose stakeholders to significant liability. As a result, vaccine cold chain technology has advanced beyond passive insulation and is now centered on active monitoring, real-time visibility, and data-backed validation. In this environment, intelligent monitoring systems are indispensable tools in safeguarding therapeutic efficacy and operational credibility.
The Vulnerability of the Vaccine Cold Chain
 Vaccines are complex biological agents that require stringent environmental control to preserve their potency. Most formulations must be maintained within tightly regulated temperature ranges, typically between 2°C and 8°C, to ensure stability and efficacy. Even brief deviations, whether they occur during storage, handling, or transit, can cause molecular deterioration and compromise both the safety and therapeutic effectiveness of the dose. The global COVID-19 vaccination effort underscored these vulnerabilities, exposing systemic gaps in cold chain infrastructure and prompting a critical reassessment of existing distribution protocols.
Vaccines are complex biological agents that require stringent environmental control to preserve their potency. Most formulations must be maintained within tightly regulated temperature ranges, typically between 2°C and 8°C, to ensure stability and efficacy. Even brief deviations, whether they occur during storage, handling, or transit, can cause molecular deterioration and compromise both the safety and therapeutic effectiveness of the dose. The global COVID-19 vaccination effort underscored these vulnerabilities, exposing systemic gaps in cold chain infrastructure and prompting a critical reassessment of existing distribution protocols.
In response, the pharmaceutical logistics sector has accelerated the adoption of advanced vaccine cold chain technology. Continuous monitoring, automated reporting, and end-to-end traceability have become baseline requirements rather than best practices. Whether managing next-generation mRNA vaccines or conventional attenuated virus platforms, cold chain systems must now demonstrate a high degree of resilience, accountability, and regulatory alignment. Additionally, the integration of real-time data analytics has enabled stakeholders to detect, respond to, and prevent thermal excursions before they compromise product integrity.
The Role of Smart Temperature Monitoring in Cold Chain Resilience
Robust packaging and insulated shippers provide foundational protection, but thermal stability alone does not guarantee compliance. Without reliable, traceable monitoring data, shippers and healthcare providers are left without proof of efficacy. This is where smart temperature monitoring becomes indispensable.
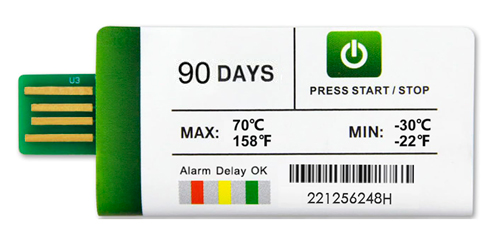
Devices like the Fresh Tag 1 USB Temperature Data Logger play a critical role in vaccine shipment monitoring. Engineered for low-cost, high-reliability performance, this single-use logger offers precision logging across a wide temperature range, from -30°C to +70°C, with an accuracy of ±0.5°C. Its waterproof, food-grade casing and IP67 protection rating ensure durability in harsh transport environments.
The Fresh Tag 1 stands out for its plug-and-play functionality. When inserted into any PC, the device automatically generates a tamper-proof PDF report without requiring any software. For organizations managing large volumes of shipments across decentralized supply chains, this feature significantly simplifies compliance and audit readiness.
Key features include:
- Up to 180 days of data logging, with configurable logging intervals from one minute to 24 hours, allowing flexibility for both short-term and extended distribution timelines
- Factory-preset parameters, reducing user setup time and ensuring consistent configuration across global shipments to meet GDP and FDA requirements
- Customizable alarm points, enabling stakeholders to define acceptable temperature thresholds tailored to specific vaccine profiles or regulatory jurisdictions
- LED visual indicators, which provide immediate, at-a-glance status updates to logistics personnel, even in low-light or high-throughput warehouse environments
- Automatic report output in PDF, CSV, or dual formats, supporting secure data transfer, audit readiness, and seamless integration into digital quality systems
- Full compatibility with Windows and Mac OS platforms, requiring no proprietary software, drivers, or connectivity accessories for access to reports and temperature logs
These capabilities make the Fresh Tag 1 an essential asset in vaccine cold storage tracking, especially for temperature-sensitive immunizations moving through long-haul or last-mile routes. Its compact, waterproof design (IP67) ensures reliable performance under varied environmental conditions, including air, sea, and overland transport. Combined with its single-use simplicity and tamper-proof data output, the Fresh Tag 1 strengthens cold chain accountability while minimizing operational burden for healthcare providers and pharmaceutical distributors alike.
Integrating Smart Monitoring into End-to-End Cold Chain Strategy
Effective cold chain management requires more than reliable monitoring devices; it demands a unified strategy that connects packaging, logistics, infrastructure, and compliance within a cohesive framework. In the vaccine and pharmaceutical sectors, where regulatory oversight is stringent and product integrity is critical, success hinges on building resilience throughout the distribution network. Nordic Cold Chain Solutions meets this need through a comprehensive, end-to-end approach that strengthens visibility, accountability, and control at every stage.
Our integrated vaccine cold chain solution includes:
- Validated thermal packaging tailored to specific vaccine types, designed to maintain required temperature ranges across various transit durations, environmental conditions, and shipping methods. These systems are tested against ISTA standards and configured for compatibility with WHO PQS and other global regulatory frameworks.
- Cold chain logistics planning and oversight, ensuring route optimization, contingency mapping, and carrier coordination to minimize risk of delays, exposure, or temperature excursions during distribution.
- Temperature-controlled shipping and storage infrastructure, including refrigerated transport units, insulated shippers, and controlled-access cold rooms, all monitored for continuous performance and traceability.
- Continuous remote monitoring and automated data reporting, enabled by smart sensors and data loggers that transmit real-time data to centralized platforms, allowing for immediate intervention in the event of thermal deviations.
- Expert consultation to ensure compliance with GDP (Good Distribution Practices), including support for documentation, SOP development, staff training, and audit preparation for global distribution networks and regulatory submissions.
Every link in the vaccine distribution chain, from the manufacturing facility to the point of administration, is analyzed and strengthened. This level of end-to-end cold chain monitoring allows for proactive issue resolution and reduces the likelihood of product loss or delays.
Integrating smart loggers like the Fresh Tag 1 into pack-outs not only verifies product viability upon delivery but also reinforces accountability throughout the logistics chain. The resulting data provides a single, verifiable record for regulatory compliance, patient safety, and stakeholder transparency.
Why Temperature Monitoring Is Essential for Secure Vaccine Transport
In a global pharmaceutical supply chain, the safe delivery of vaccines depends on continuous, data-driven visibility. With products that are thermally fragile and highly regulated, stakeholders cannot afford uncertainty during transit. As vaccine shipments move through complex logistics networks, real-time oversight becomes essential to safeguard against degradation, noncompliance, and operational failure.
Without smart monitoring tools in place:
- Vaccine degradation may go undetected, allowing compromised doses to reach healthcare providers or patients
- Chain of custody becomes fragmented, increasing the likelihood of handling errors and legal exposure
- Regulatory audits grow more complex and risk-prone, delaying distribution and eroding regulatory confidence
- Public confidence is undermined, especially during mass immunization campaigns
With smart temperature loggers such as the Fresh Tag 1:
- Real-time condition monitoring enables proactive response, identifying and addressing excursions before integrity is compromised
- Critical temperature breaches trigger immediate alerts, thanks to customizable thresholds and LED indicators
- Detailed, timestamped data supports performance reviews, improving route planning and carrier selection
- Automated documentation streamlines compliance, providing tamper-proof PDF or CSV reports for regulatory use
Smart monitoring doesn’t just protect the product; it reinforces the integrity of the entire vaccine delivery ecosystem. By deploying tools like the Fresh Tag 1, healthcare systems and supply chain operators strengthen their ability to maintain compliance, ensure therapeutic efficacy, and uphold public trust, while also enabling more agile and resilient vaccine access worldwide.
Ensuring Compliance, Protecting Public Health, and Scaling with Confidence
Cold chain failures have far-reaching consequences that go beyond operational setbacks. They directly impact public health, particularly in global vaccination campaigns where temperature excursions can compromise the efficacy of life-saving immunizations. As distribution networks scale to serve vulnerable and underserved populations, maintaining thermal integrity through reliable vaccine cold chain technology is critical.
The Fresh Tag 1 USB Temperature Data Logger supports this mission by helping organizations comply with international standards, including WHO and CDC cold chain protocols, FDA guidelines for biologics, EU GDP regulations, and EN12830 certifications for data logger performance. Its preset parameters, automatic reporting, and intuitive interface reduce logistical complexity and enable confident deployment at scale.
Designed to meet the demands of high-volume distribution environments, the Fresh Tag 1 is lightweight, single-use, and cost-effective, making it ideal for national rollouts, retail pharmacy chains, and humanitarian aid programs. Its plug-and-play functionality and broad operating system compatibility eliminate the need for IT-intensive setup, allowing global health organizations, regional providers, and commercial pharma companies to strengthen compliance and expand reach without added burden.