Every December, vaccine demand rises sharply. Flu season peaks just as millions of people prepare to travel, gather indoors, and interact in settings that accelerate the spread of respiratory illnesses. Pharmacies, clinics, and hospitals brace for higher patient volumes, while distributors race to meet surging orders. This convergence of flu season and holiday mobility makes December one of the most critical months for vaccine distribution.
Amid this heightened activity, the vaccine cold chain faces extraordinary stress. Warehouses, transportation providers, and last-mile delivery networks must operate with absolute precision to keep every dose within strict temperature ranges. A single lapse can compromise efficacy, placing both public health and organizational reputations at risk.
The Fragility of Vaccines
Vaccines are not like standard pharmaceuticals. They are biological products with narrow tolerances for heat, cold, and time outside controlled environments. Even short-term deviations can trigger degradation, leaving patients unprotected. For many vaccines, especially those formulated with mRNA or protein-based technologies, the margin for error is razor-thin.
December exacerbates this challenge. Transportation networks are congested, weather conditions unpredictable, and fulfillment timelines compressed. With every potential stressor magnified, vaccine cold chain management becomes not just a compliance requirement but a public health safeguard.
Why the Cold Chain Matters More in December
Several factors make the holiday season a particularly vulnerable period for vaccine logistics:
1. Increased Demand:
 Pharmacies and clinics must stock higher volumes of influenza, COVID-19, and other vaccines, creating pressure on inventory management and storage capacity.
Pharmacies and clinics must stock higher volumes of influenza, COVID-19, and other vaccines, creating pressure on inventory management and storage capacity.
2. Compressed Timelines:
Patients seek vaccinations before travel or family gatherings, driving urgent shipping and faster turnaround expectations.
3. Strained Logistics Networks:
Holiday parcel volumes can overwhelm carriers, increasing the likelihood of delays or mishandling.
4. Variable Environmental Conditions:
Shipments may be exposed to cold snaps, snowstorms, or unheated transit points that complicate thermal stability.
Together, these pressures make it essential to deploy validated, design-tested solutions that remove guesswork and ensure reliability.
Nordic’s Approach to Vaccine Cold Chain Management
At Nordic Cold Chain Solutions, we specialize in protecting vaccine integrity under precisely these kinds of high-stakes conditions. Our systems are engineered and validated to meet the rigorous requirements of medical cold chain logistics, particularly during peak seasonal demand.
Key pillars of our approach include:
- Industry-Leading Packaging: From frozen parcel shippers to insulated containers, each product is tested to maintain stability across a range of durations and payloads.
- Comprehensive Planning: We work with healthcare providers and distributors to anticipate seasonal demand surges and design scalable fulfillment strategies.
- Advanced Monitoring: Temperature indicators and monitoring devices provide real-time visibility, ensuring every shipment arrives within the validated range.
- Expert Consultation: Our team of cold chain specialists supports clients with training and best practices for holiday readiness.
This integrated model transforms cold chain management from a reactive safeguard into a proactive strategy for public health protection.
Design-Tested Frozen Vaccine Shippers
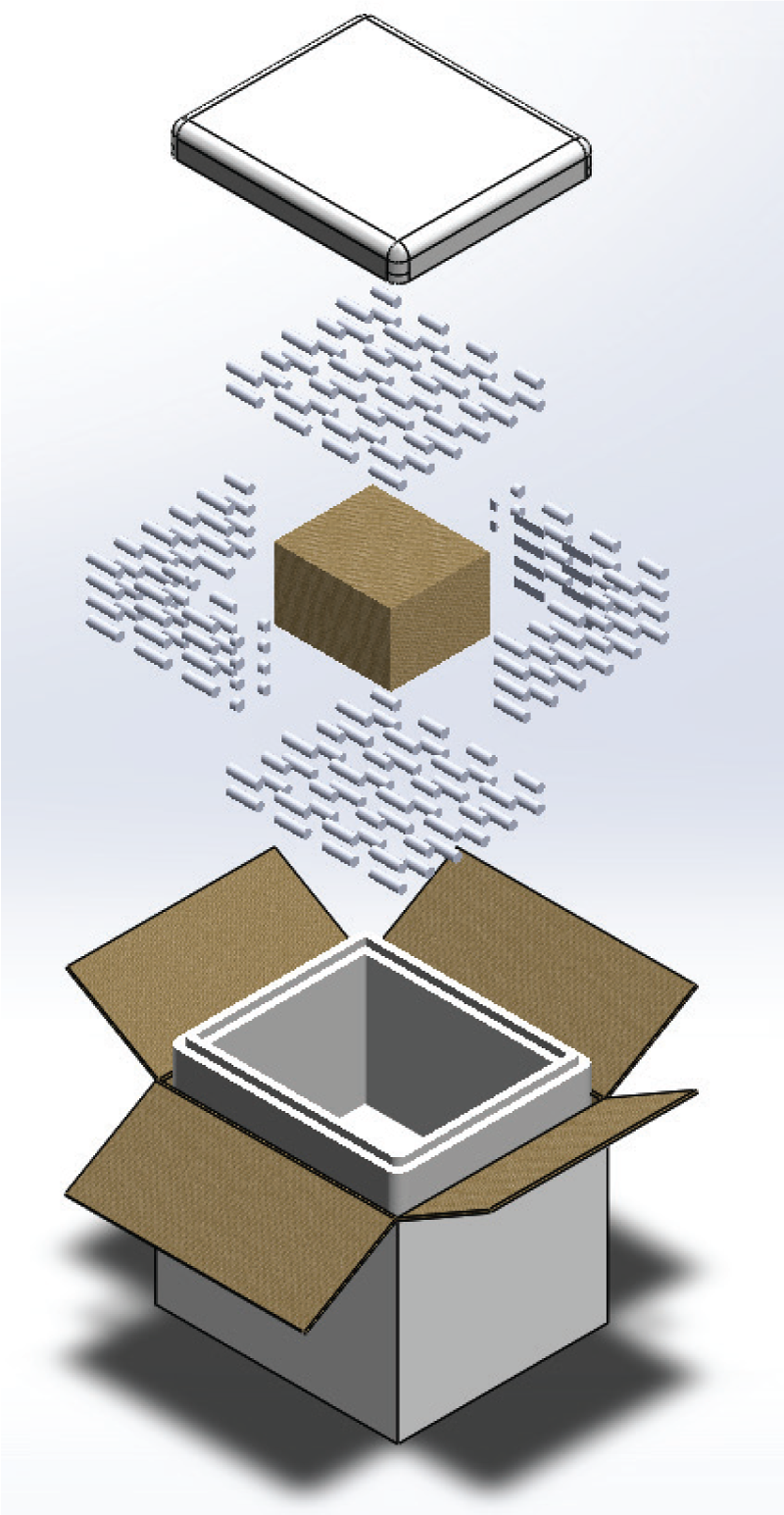 Central to our holiday vaccine solutions are Nordic Express Design-Tested Frozen Shippers. Built to ISTA 7E standards, these systems have been validated to withstand the stressors of real-world distribution, including delays, variable handling, and environmental extremes.
Central to our holiday vaccine solutions are Nordic Express Design-Tested Frozen Shippers. Built to ISTA 7E standards, these systems have been validated to withstand the stressors of real-world distribution, including delays, variable handling, and environmental extremes.
Available in sustainable insulation and EPS configurations, these shippers provide flexibility across payload sizes and duration requirements. Their multi-component designs ensure that vaccines remain frozen for one- or two-day shipments, making them ideal for urgent seasonal deliveries.
By combining predictable refrigerant performance with durable insulation, our frozen shippers reduce the risk of temperature excursions, even when carriers face holiday congestion. This proven reliability allows healthcare providers to maintain vaccination schedules with confidence.
Safeguarding Public Health Through Reliability
The consequences of failure in vaccine cold chain management are significant. A single compromised shipment can lead to:
- Reduced Vaccine Efficacy: Exposed doses may lose potency, leaving recipients vulnerable.
- Financial Waste: Destroyed shipments translate into both direct losses and delayed vaccination programs.
- Regulatory Exposure: Compliance failures carry risks of fines, recalls, and reputational harm.
- Public Health Setbacks: Most importantly, compromised vaccines undermine community immunity during the time it is most needed.
During the holidays, these stakes are amplified. By maintaining validated cold chain performance, Nordic helps safeguard not only the integrity of vaccines but also the trust of communities counting on them.
Operational Efficiency Under Seasonal Strain
Reliability is only part of the equation. Distributors also need systems that allow them to scale rapidly without sacrificing compliance. Nordic’s vaccine packaging solutions are designed with operational efficiency in mind.
- Pre-configured Packouts: Simplified workflows reduce training requirements and minimize errors during high-volume periods.
- Space-Saving Formats: Compact insulation systems lower warehouse storage needs and reduce dimensional shipping costs.
- Scalable Kits: Standardized components streamline inventory management and allow distributors to handle surges with consistency.
By integrating reliability with efficiency, our solutions empower healthcare organizations to keep pace with seasonal surges while staying aligned with regulatory requirements.
Preparing for the Next Surge: A Holiday Imperative
The pressures of December highlight why resilient vaccine cold chain strategies are essential year-round. Organizations that strengthen their systems during the holiday surge are better equipped to handle the unexpected, from future flu seasons to pandemic responses and new vaccine launches. By partnering with Nordic, providers gain more than packaging, they gain a trusted advisor who can navigate regulatory complexity, seasonal surges, and long-term planning.
As flu season collides with holiday travel, the stakes could not be higher. Rising demand, compressed timelines, and strained logistics make December the ultimate test of cold chain resilience. Nordic Cold Chain Solutions delivers validated packaging, advanced monitoring, and expert guidance to ensure every vaccine arrives with uncompromising quality. Now is the time to reinforce your vaccine cold chain strategy.






